
After attending the 2-day summer Ohio Corn & Wheat-sponsored Feed the World workshop, Matt McCormick presented the Fermentation in a bag lab and the Distillation of ethanol from corn lab to his students. Both labs integrated well with the Intro to Chemistry unit in his Physical Science class, as they support the study of chemical reactions and separation of chemicals through physical means.

McCormick started with the Fermentation in a bag lab as an introduction for the distillation lab. “We began with a pre-lab discussion as to what yeast actually is, how it is used for baking and fermenting materials, and what amylase and glucoamylase enzymes do. Students worked in pairs to monitor bags and collect data.
The math teacher collaborated on the data aspect of the lab, with students inputting the data results of bag height versus time into the DESMOS app. This app lets students analyze graphical data including slope, R-value, and Y-intercept. With that information, the students were able to write the equation for the line and to construct a simple X vs. Y line graph.
McCormick said, “We shared group data to the front board so that all students could create graphs for bags A, B, C, and D. The next day we analyzed the graphs to determine that bag D, with both amylase and glucoamylase, produced the most CO2. This shows how important the use of both enzymes are in the process of ethanol production and the reduction of the long-chained sugar molecules.”
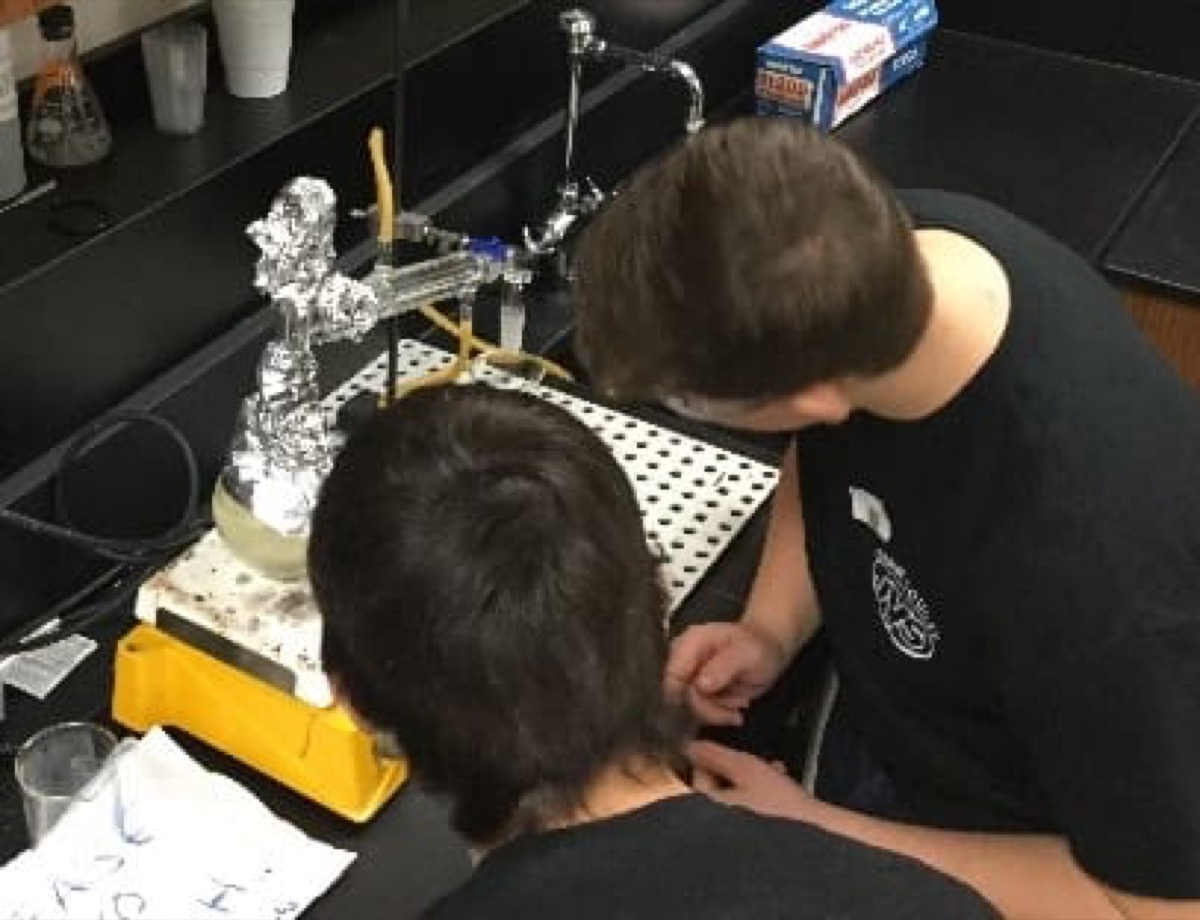

Possibly due to problems with the yeast, McCormick had some difficulties with distillation, resulting in low yield and quality. “We finally got three beakers that provided enough ethanol to at least provide a nice blue flame; however, the batches were not concentrated enough to run the Stirling engine.”
Prior to conducting the ethanol lab, McCormick presented a powerpoint showing the process used to extract ethanol from corn as well as other products that can be made with the by-products. Students asked questions such as; why don’t we use pure ethanol in cars? What vehicles benefit from E-85? “We had good discussions about the reasons why certain vehicles can’t tolerate ethanol in their fuel systems and how the auto manufactures have created flex fuel vehicles to run on E-85.”
McCormick said he will continue to use these lab activities next year and beyond. “These were great hands-on activities. The distillation lab, although complex, showed a real-world example of separation of mixtures by physical means. The students really enjoyed seeing the ethanol burn on the lab counter top—they were mesmerized by the blue flame burning in the darkened room.”